by Lisa Mesrop
With their lumpy and warty features, large animated eyes, and soft-padded toes, toads are pretty darn cute. But there is one group of cute and slimy toads you don’t want to kiss. Toads within the family Bufonidae produce a poison cocktail from specialized glands in their skin that is potent enough to kill large predators and even human adults. Toads use their poison for defense and protection against pathogens.
Reference: Lenaerts C, Wells M, Hambÿe S, Blankert B. Marinobufagenin extraction from Rhinella marina toad glands: Alternative approaches for a systematized strategy. J Sep Sci. 2019 Apr;42(7):1384-1392. doi: 10.1002/jssc.201800879. Epub 2019 Feb 5. PMID: 30667156.
Dr. Jennifer Stynoski’s amphibian research group at the University of Costa Rica (UCR) is interested in understanding how poisonous toads acquired their poison glands during development and in evolution. Dr. Stynoski’s research group is part of the Laboratory for the Investigation of Dangerous Animals (LIAP) at the Codomiro Picado Institute of UCR, which is focused on understanding the chemical ecology of many different types of venomous and poisonous animals.
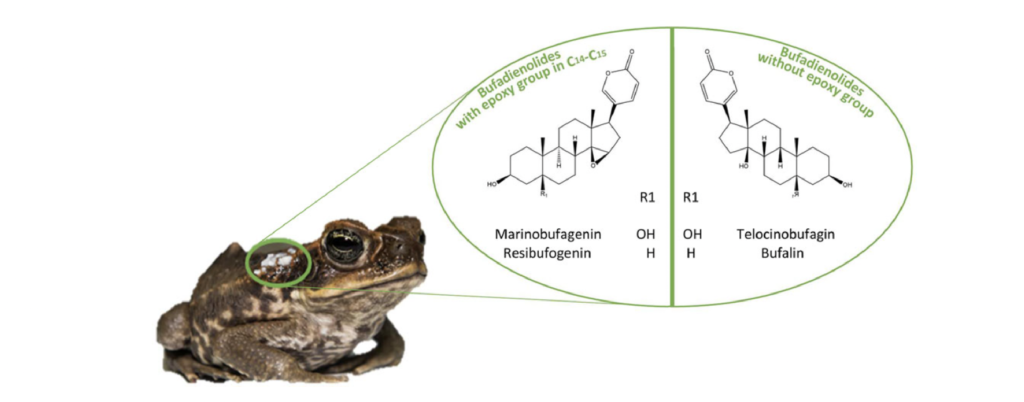
Dr. Stynoski uses an integrative approach and draws upon tools and knowledge from different disciplines – evolutionary biology, biochemical ecology, developmental and molecular biology – to understand how toads develop poison glands in their skin and produce different types of poisons. One specific family of poisons called Bufadienolides has a well-documented role in medical and pharmaceutical practices. Bufadienolides are classified as steroids, and there are over 200 types of known Bufadienolides that are produced by different toad species within the family Bufonidae.

Historically, toad poison extract has played an important role in traditional medicine. For example, the Chinese drug “Chan’Su” is derived from toad poison extract and has been used to treat cardiac dysfunction and cancer due to its anti-tumor activities. In addition, Bufadienolides are also used as therapeutic agents (anti-fungal and anti-protozoan) to treat common human parasitic diseases and amphibian fungal diseases. However, isolating large quantities of Bufadienolides from natural toad populations is challenging and harmful to the ecosystem. First, Bufadienolides is difficult to extract and purify from toad skin. Second, harvesting these compounds from natural toad populations can have detrimental impacts on toad populations in the wild, which are already in decline due to a toad-related fungal disease called chytridiomycosis.

In order to synthesize Bufadienolides and anti-venom in the lab without using toads, we need to first understand how toads make these compounds themselves. Dr. Stynoski and her research team are taking an evolutionary comparative approach to identify the genes involved in the biosynthesis of Bufadienolides. The chemical structure of Bufadienolides is similar to toxin compounds produced by plants and fireflies, but whether the underlying genes involved in their biosynthesis are related by common ancestry or have independently evolved is unknown. Dr. Stynoski says, “In theory, there are a lot of different ways to do that pathway, but whether they are conserved or convergent is still up for grabs.”
Their current working hypothesis is that Bufadienolides are derived from the sex hormone, progesterone, but the enzymes involved in the conversion of progesterone to the different types of Bufadienolides are unknown. Their integrative approach will characterize key genes in the biosynthesis pathway of Bufadienolides and link them to the novel cell types that express them in the Toad’s skin. The results generated by her research team will not only have revolutionary implications for understanding evolutionary innovations, but also on toad conservation efforts by providing a new pharmaceutical platform for which these compounds can be synthesized, and not harvested from natural toad populations.
Calls of many frogs and toads on egg collection night


