
Chameleons use skin coloration to broadcast mood or intent: aggressive males might flash darkly at nearby intruders, while females swooning over suitors might blush a brilliant pink. Because of how important visual communication is to chameleons, they rely very heavily on their visual sensory system. Their optical specializations include the ability to focus their gaze in two separate directions at once and the ability to see in the ultraviolet.
If humans were built like chameleons, buying sunglasses might get tricky: our eyes would be the size of basketballs, each bigger than our brains.
Such is the case for the chameleon, whose eyes easily dwarf its brain. A chameleon’s eyes are so big, in fact, that they seem to have displaced the brain entirely, rotating it nearly 90 degrees. This surprising finding about chameleon brain morphology was presented by Daniel Hughes, then a PhD candidate at the University of Texas at El Paso (UTEP), at the 2018 SICB Annual Meeting in San Francisco, California.
This discovery results from recent efforts to develop methods for preserving the brain tissue of wild-caught African chameleons by Hughes; Arshad M. Khan, the director of the Systems Neuroscience Laboratory at UTEP; and their collaborators. Although there is a robust body of literature detailing the neuroanatomy of species like laboratory rodents, the brains of chameleons – and the brains of most other reptiles for that matter – are black boxes to the scientific community.
It was in part due to the challenges of working with non-model and exotic species, Hughes said, that no one had formally described a technique to preserve the brain of a wild-caught chameleon. For one, chameleons are simply difficult to reach. The transatlantic flight to Africa is grueling in itself, but the journey only becomes more arduous once on land. Hughes and his adviser, Eli Greenbaum, an evolutionary biologist at UTEP, know these struggles firsthand, having trekked to the Congo Basin and the Albertine Rift of Central Africa multiple times in search of chameleons. In order to reach the remote sites, the researchers must slowly navigate roads riddled with potholes, and they were lucky if they could successfully traverse 10 kilometers per day. On Hughes’ first trip, their field truck frequently got stuck in muddy ditches, stranding them for hours at a time. At one point, the truck even flipped over.
Chameleon brains can also be difficult to preserve. Tissue preservation, especially by a process called fixation, is a crucial first step for neuroanatomical studies. In fixation, tissues are flooded with chemical fixatives like formalin, which protect and stabilize the tissue, preventing decay. Traditional fixation methods require laboratory equipment and resources – -20°C cold storage, ice-cold reagents, fume hoods, and electricity-powered pumps- that are impossible to come by in the remote locations these African chameleons typically inhabit. Local regulations of many foreign countries prohibit the exportation of live chameleons to international labs, and so scientists will need to perform procedures on-site. Many of Hughes’ and Greenbaum’s field sites don’t have reliable electricity, however, let alone fancy freezers.

Nevertheless, the researchers were determined to try to collect these perishable data, not only to begin cataloguing chameleon neuroanatomy, but also to help add uncommon datasets to natural history museum collections. Museum collections are an important tool for documenting current and historic patterns of biodiversity. But while some things are easily collected-DNA, birdsongs, and the physical specimens themselves, for example-the brain has not been one of them, leading to a woeful underrepresentation of neural biological diversity in museum collections. This could have dire consequences. “[We could lose] a potentially really rich dataset to understand the brain from an evolutionary perspective,” said Hughes.
Yet not so long ago, collecting DNA from field specimens was unusual; today, DNA collection is as routine as recording an animal’s age and sex. Developing methods for preserving brain tissue in the field could well become an additional tool in a field biologist’s toolkit. To Hughes and his collaborators, a logical and important task was to modify laboratory-based fixation protocols for field conditions.
The researchers substituted common laboratory equipment for the resources they had at hand, sedating chameleons in Tupperware and storing formalin in old water bottles. Instead of relying on an electricity-powered peristaltic pump for a method called perfusion fixation, where a needle is inserted into an animal’s heart and a pump is used to steadily introduce fixative into the animal’s circulatory system, Hughes performed this process by hand, injecting two small syringes of formalin as steadily as possible into the tiny chameleon hearts.
Their work resulted in a set of well-preserved chameleon brains-but the real test would be whether these brains were viable specimens. Would the quality be sufficient for use in detailed neuroanatomical experiments?
At the Systems Neuroscience Laboratory at UTEP, the researchers prepared a control set of brains taken from chameleons purchased from pet shops and fixed using traditional laboratory methods. They then performed a suite of common staining and immunochemistry experiments-which allow scientists to visualize the microanatomy and physiology of the brain-on a set of field-fixed brains and a set of lab-fixed brains. The scientists sectioned the brains into thin slices, stained slices with different antibodies and chemicals, and visualized the slices under a microscope. They found that their field-produced brains were indistinguishable from control brains.
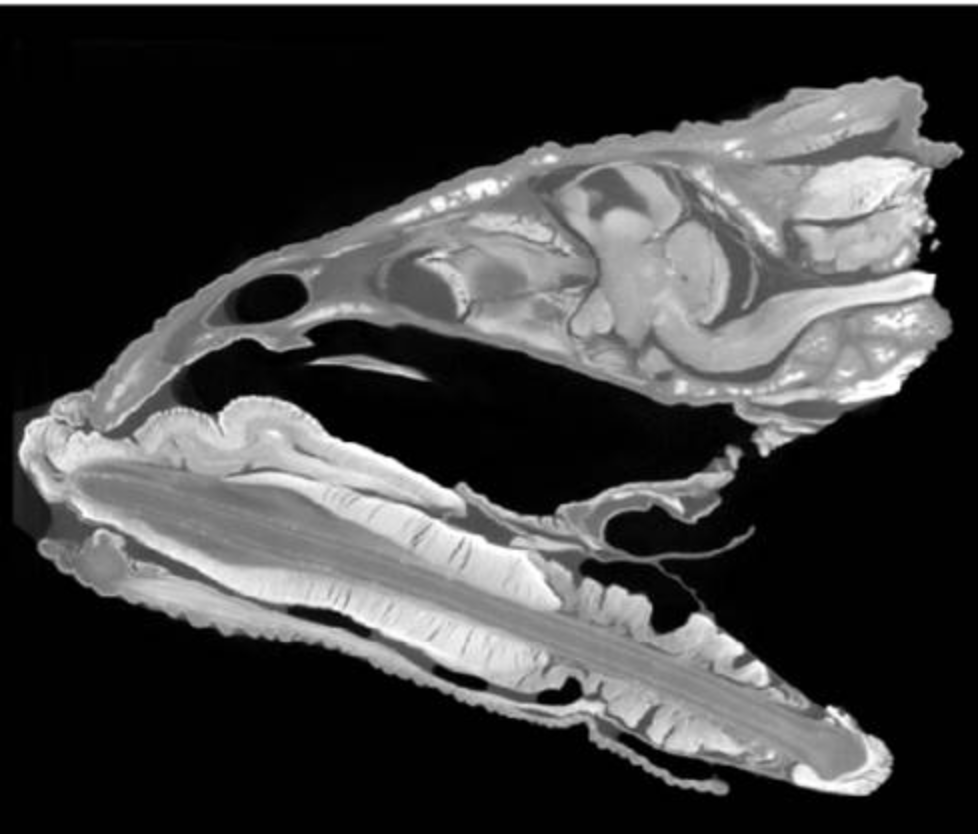
The scientists had also “drop-fixed” a set of chameleon heads: after euthanizing a chameleon, they removed its head and immersed it in a formalin solution, preserving brain tissue alongside muscle, glands, and skull. With this collection, Paul M. Gignac, an anatomist at Oklahoma State University Center for Health Sciences, performed Diffusible Iodine-based Contrast-enhanced Computed Tomography-or diceCT-a method that allowed them to simultaneously visualize soft tissues and bony structures, leaving the entire head intact. Once again, their field-produced samples were indistinguishable from control samples. More astounding, this method led to their discovery that the chameleon brain is rotated in its skull.
What is most exciting about this finding, said Hughes, is that it didn’t take much to discover, and it required minimal supplies and training. The researchers simply modified and applied common methods to a species that had not yet been studied before, in the process gaining invaluable information about the organism’s neuroanatomy.
The ability to use these methods on exotic species in remote locations may also have important consequences for understanding biological diversity, Hughes said. There are many more discoveries to be made-but these are time-sensitive. In the past 200 years, almost 200 vertebrate species have gone extinct, a rate that is not slowing; indeed, a recent study found that 32% of known vertebrate species are decreasing in both population size and range (Ceballos et al., 2017).
For Hughes, this disastrous and rapid loss of biodiversity fuels his desire to document understudied organisms.
“We’re losing species at an incredible rate,” Hughes said, “and not only should we be trying to discover and explore everything in the natural world, [but we should also] consider nontraditional types of datasets or traits that aren’t easily preserved in museum specimens.”
Field methods

In the Rwenzori Mountains National Park of Uganda, Owerangi Enock, a local guide and interpreter raises a stick to a tree in the hopes of pulling down a chameleon. Photo courtesy of Danny Hughes.
The easiest way to catch a chameleon is to locate them in the evening while they’re asleep, said Hughes. The chameleons cling to tree branches as they slumber, and their skin turns a pale yellow or light green. Hughes takes a flashlight or headlamp and scans the trees for chameleons. When a chameleon gets illuminated with a flashlight, its skin flashes brilliantly against the dark greenery-a well-populated tree looks like it’s bedecked with Christmas lights.
When Hughes spots a chameleon perched on a tree, sometimes as high as 20 meters up, he raises a stick to the perch and gently prods the sleeping chameleon. Sometimes the chameleon wakes up and groggily climbs onto the stick-Hughes immediately lowers the stick, chameleon in tow. The alternative is less elegant: if the chameleon doesn’t budge, he tosses smaller sticks at the perch until the chameleon is jostled and tumbles gently from the branch into his hands.


Congolese roads vs field truck


diceCT section
External Links
http://theconversation.com/natural-history-collections-are-fine-specimens-of-great-value-16353
Ceballos, G., Ehrlich, P.R., Dirzo, R., 2017, Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proceedings of the National Academy of Science of the United States of America. 114(30) E6089-E6096.
By Tracy Burkhard, University of Texas at Austin

